Getting Elemental: What’s Behind the Rare Earth Headlines
A quick tour through the rare earths — the elements behind the deal that never happened
Rare earth elements have taken center stage in recent days, emerging as a new fault line in global politics. On February 28, 2025, Ukrainian President Volodymyr Zelensky met with President Donald Trump at the White House to finalize a deal granting the US access to Ukraine’s rare earth deposits—critical minerals essential for high-tech applications—as partial repayment for US military aid in Ukraine’s war with Russia. The meeting ended abruptly without a deal, underscoring the increasingly tangled relationship between natural resources, diplomacy, and global power struggles.
Adding to the confusion, media reports have often conflated rare earth elements with the broader category of critical raw materials. In 2022, Ukraine’s Deputy Environmental Minister said 5% of the world’s critical raw materials lie within Ukraine, which covers just 0.4% of Earth’s surface. Many news outlets mistakenly applied this figure to rare earth elements specifically—a textbook example of scientific nuance getting flattened into a convenient headline.
To clarify, rare earth elements are just a subset of the 50 critical raw materials listed by the US Geological Survey. Of those, 16 are rare earths (only promethium is absent), and their importance stretches far beyond today’s headlines.
Periodic table to the rescue
The fact that President Trump was pushing to secure these resources is worth noting. To understand why, we need to step back and follow the fascinating history of the periodic table, and how it shaped the technology we rely on today.
For centuries, humans tried to organize naturally occurring elements in a manner that was logical. In the sixteenth century, Theophrastus Bombastus von Hohenheim, or Paracelsus, outlined the tria prima based on the prevailing influence of alchemy. He presented a triangle with vertices represented by mercury, sulphur, and salt, believing all of life could be reduced to a combination of these three parts. Parcelsus's tria prima could perhaps be viewed as the crudest attempt at a periodic table of elements.
Chemistry began to emerge as a distinct science, separate from alchemy, thanks to Robert Boyle's work in the late seventeenth century. Over the next 100 years, substantial efforts were made to organize the elements. Antoine-Laurent Lavoisier defined elements as being substances that cannot be broken into simpler entities. Based on his definition, he considered 33 substances to be elements and published the book Traité élémentaire de chimie in 1789 to spread his thesis. (Here is a 1789 English translation by Robert Kerr - some of the points Lavoisier makes in the preface are brilliant and timeless). Through his calculations, he predicted the existence of silicon—which was discovered 40 years later. Thanks to subsequent refined techniques, we now know that ten of the 33 entities he called "elements" are not elements.
Dmitry Ivanovich Mendeleyev, or Dmitri Mendeleev, was born almost 40 years after Lavoisier's publication. By this time, John Dalton had published A New System of Chemical Philosophy (1808), listing 20 elements with their atomic weights. Fast forward another 50 years when at an international conference of chemists, it was decided that hydrogen which was known to be the lightest element be given a weight of 1, and the weight of all other elements would be ascribed based on how many times heavier they were than hydrogen. Soon after, British chemist John Newlands noticed a repeating pattern in atomic weights—what he called a "law of octaves," likening it to music. Elements seemed to fall into groups of 7, repeating every 8th element. But this pattern didn't hold beyond a point, and his work was ridiculed. Other chemists continued to work on this problem, and unaware of each others' work, Mendeleev and Julius Lothar Meyer came up with their own versions of how the elements are organized.
On March 6, 1869, Dmitri Mendeleev presented the first periodic table to the Russian Chemical Society. His table, titled “The Dependence between the Properties of the Atomic Weights of the Elements”, was a triumph of scientific intuition. By organizing elements according to atomic weight and chemical behavior, Mendeleev revealed a hidden order in nature—and even predicted the existence of elements not yet discovered.
Less than a decade later, on March 7, 1876, Alexander Graham Bell was awarded a patent for the telephone—a device whose success depended on the chemical properties of the materials used to make it. Bell’s early phone was simple, relying mainly on iron, copper, and zinc.
Every improvement since—from rotary dials to touchscreens—has been shaped by the discovery and application of new materials, a process made vastly more efficient thanks to the periodic table. Broadly speaking, our ability to predict the properties of materials—rather than just experimenting blindly—owes everything to Mendeleev’s work. In fact, most of the advances we see in almost every facet of life—from your favorite mobile device to the discovery of new drugs—are made more efficient thanks to the periodic table.
Mendeleev's contribution was most remarkable in the table he outlined because he left gaps for elements that had not been discovered at the time, but were predicted to be found based on his calculations. The elegance of Mendeleev's work is also underscored by the fact that as we learnt more about atoms in the twentieth century, those findings in no way disturbed Mendeleev's periodic table of elements. In 1869, when Mendeleev published his periodic table, only 60 elements were known. Today, we have 118 elements (including natural, and synthesized) in the periodic table, and Mendeleev's organization remains largely intact.
Rare earth elements
Scientists knew some elements were still missing, but isolating them was another story—especially the rare earths, which stubbornly refused to stand out. They occupied an awkward middle ground: not precious like gold, not workhorse metals like iron, just oddballs hiding in plain sight.
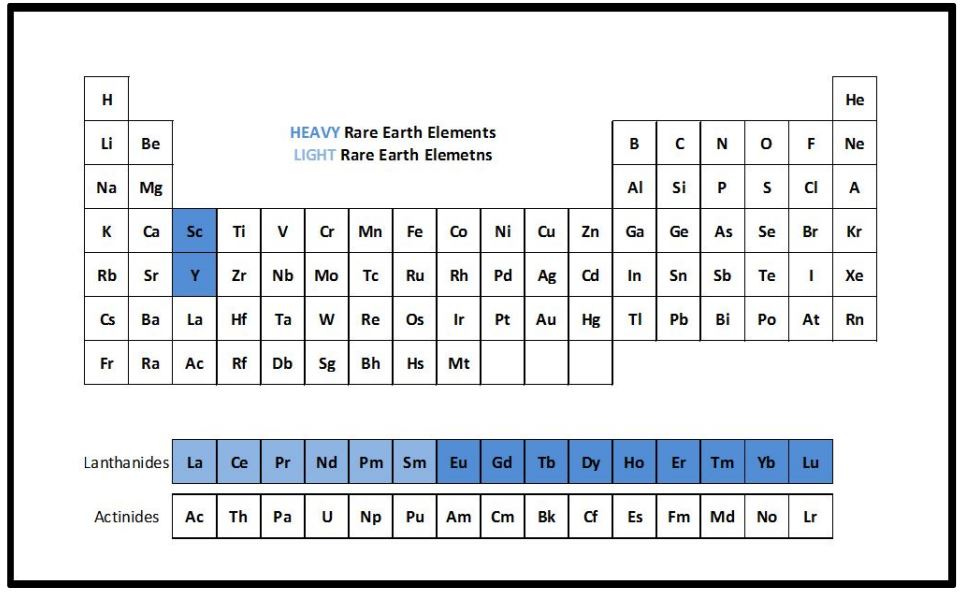
There are seventeen rare earth elements (REEs) neatly visualized in the periodic table. If you aren’t familiar with the periodic table, the rows are called periods, and columns are called groups. Elements are represented by one or two letters, mostly based on greek or latin names. Without getting too deep into the table, the image below shows the seventeen REEs - from group 3 - scandium (Sc), yttrium (Y), and the lanthanides with lanthanum (La) being a placeholder below yttrium, for the entire family commonly called lanthanides or lanthanoids.
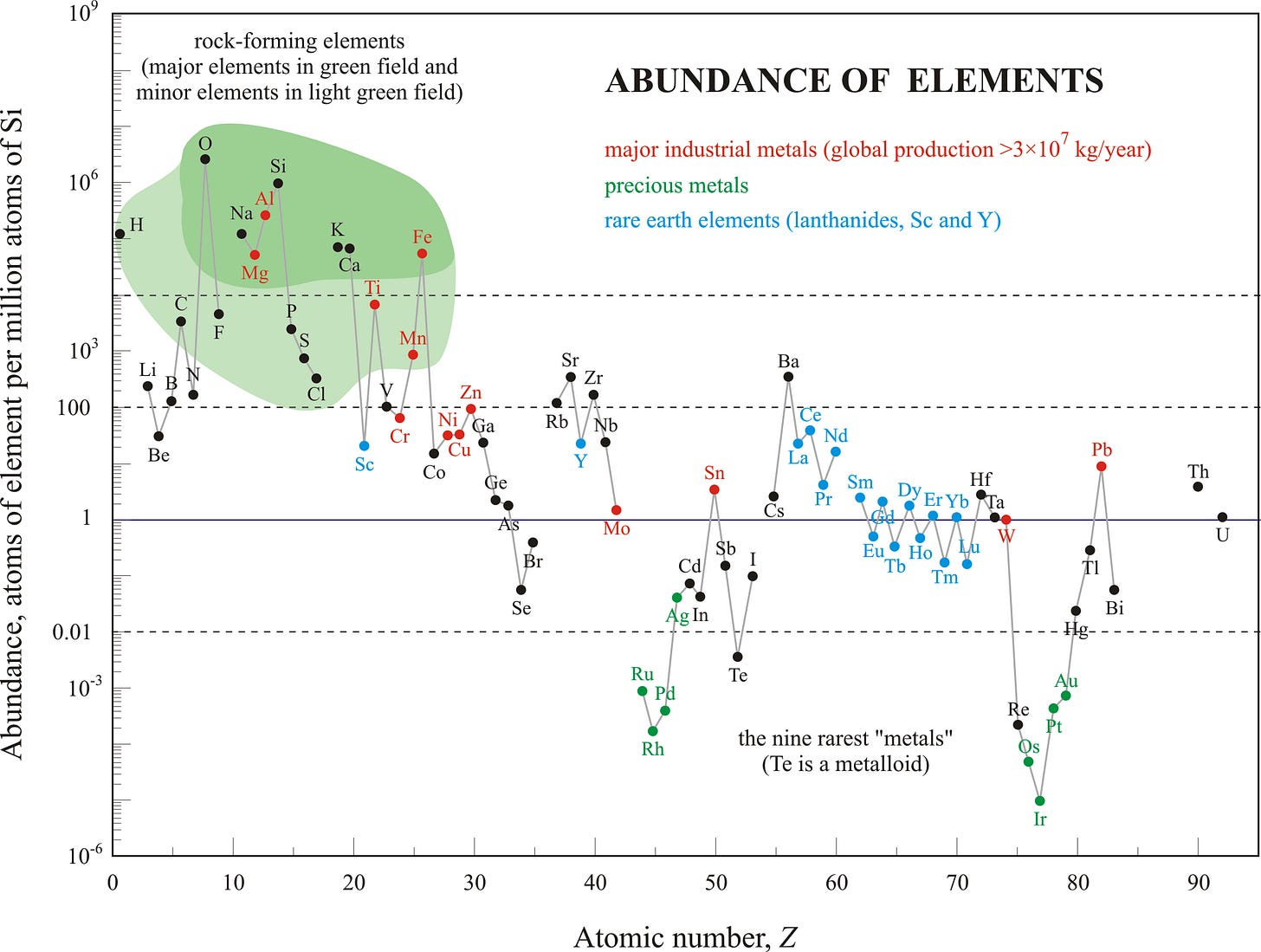
Even the name “rare earths” is misleading. The term dates back to the late 18th century and they aren’t particularly rare (some are more common than copper, and together, almost 200 times more abundant than gold). Unlike many other elements, they don’t cluster into rich, convenient veins and are instead, scattered thinly across the earth’s crust, making extraction a chemically difficult process—a challenge that delayed their large-scale use until the 20th century.
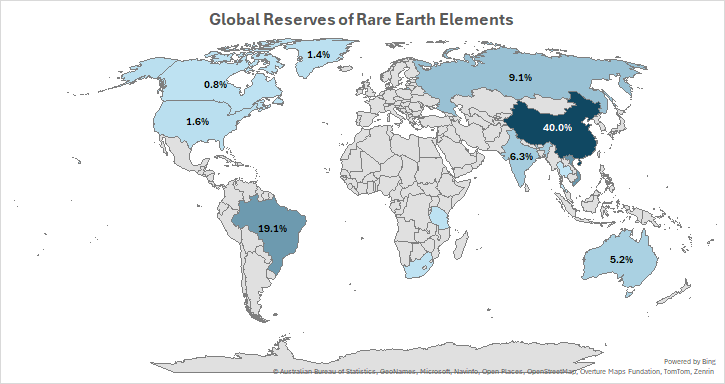
While not rare, these elements aren’t uniformly distributed—China holds the largest reserves, dictated by specific geological environments. Importantly, for these elements to be useful, they must go through a complex purification process—and today, China handles most of the world’s production, leaving the US 100% reliant on imported rare earths. In 2017, the US Department of Energy presented a report to Congress, discussing the future of alternate avenues to secure necessary amounts of rare earths. According to the 2024 report from the US Geological Survey, the China holds almost 40% of the global reserves. It’s worth noting that minable concentrations of rare earths are much lower than their overall abundance in Earth’s crust.
Why is there such a demand for rare earths?
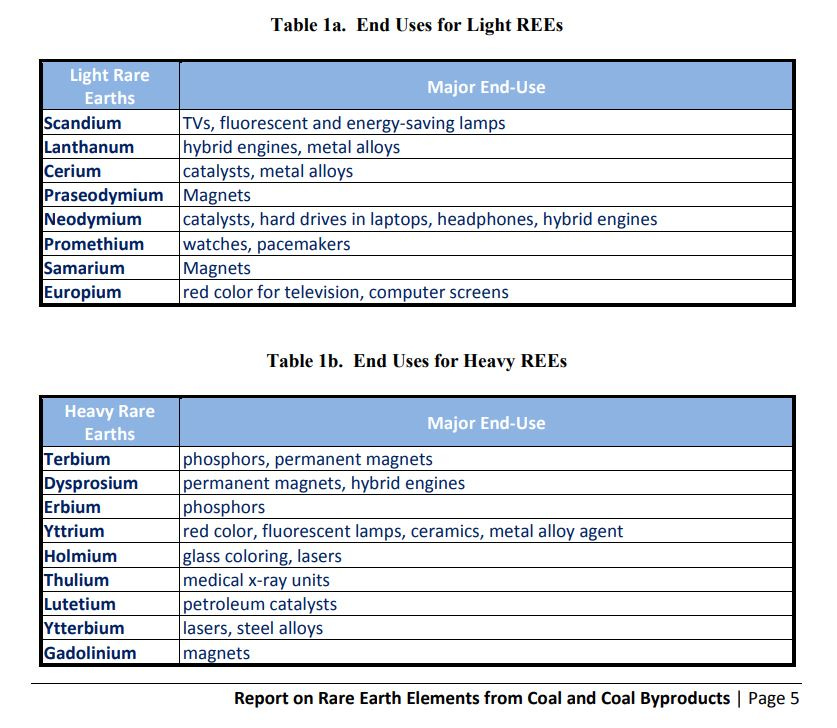
That Congressional report includes a useful summary highlighting at least one key application for each of the seventeen rare earths. It becomes clear from this summary that there is almost nothing you do or use daily is untouched by rare earths.
As we grapple with the complexities of our modern world, the critical role of rare earth elements in shaping the geopolitical landscape cannot be overstated. The competition for these vital resources has forged new alliances and intensified existing rivalries, underscoring the interconnectedness of science, technology, and global diplomacy. By understanding both the history and the current geopolitics surrounding rare earth elements, we gain a clearer sense of their significance—and the challenges ahead. Ultimately, our ability to innovate and collaborate across borders will determine how we navigate this ever-evolving landscape and ensure a sustainable and prosperous future for all.